X-linked inheritance occurs fairly frequently in retinitis pigmentosa, and in these cases the condition is sometimes referred to as X-linked RP, or XLRP. Choroideremia is also an X-linked condition (see Why genes matter).
X-linked conditions affect males far more frequently than females. Females have two X chromosomes, so even if one of them contains a faulty gene, its healthy counterpart on the other X chromosome can often compensate and sight remains unaffected. Males have only one X chromosome, and one Y chromosome that contains very little genetic information. If their X chromosome contains a faulty gene, there is no second copy to compensate, and they will develop sight loss. If there are a number of men and boys in an extended family who are living with sight loss, but no affected females, then X-linked inheritance is highly likely.
There are situations in which X-linked conditions can affect females. Sometimes the healthy copy of the X chromosome isn’t able to compensate for the faulty copy, and females can then develop a milder form of sight loss, or in some cases can be equally as affected as males.
A genetic counsellor can discuss family history with you and try to ascertain whether or not your family is affected by X-linked inheritance. A genetic counsellor can also discuss genetic testing with you. A genetic test can often identify exactly which gene is causing the problem and confirm the inheritance pattern. Genetic testing and Genetic counselling, explain more about how to access these services.
Even if someone has transitioned from their assigned gender at birth, their sex chromosomes will not change; genetic counsellors will therefore need information about birth gender for discussions around inheritance patterns and interpretation of test results.
What are the risks to my children if I am a male with X-linked retinal disease?
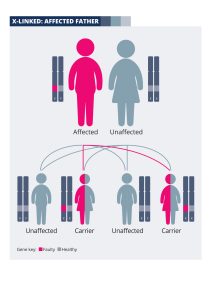 If you are a man living with an X-linked condition, you will not pass the faulty gene onto your sons. This is because they will inherit your Y chromosome, not your X chromosome. Your daughters will inherit your X chromosome containing the faulty gene. However, they will also inherit an X chromosome from their mother, which is highly likely to contain a healthy copy of the gene. Your daughters will therefore be “carriers” of the genetic fault; in most cases their sight will be unaffected, although it is possible that they will develop symptoms. A genetic counsellor will be able to discuss the risks with you in more depth. The diagram explains how the faulty gene is passed on from men with X-linked conditions.
If you are a man living with an X-linked condition, you will not pass the faulty gene onto your sons. This is because they will inherit your Y chromosome, not your X chromosome. Your daughters will inherit your X chromosome containing the faulty gene. However, they will also inherit an X chromosome from their mother, which is highly likely to contain a healthy copy of the gene. Your daughters will therefore be “carriers” of the genetic fault; in most cases their sight will be unaffected, although it is possible that they will develop symptoms. A genetic counsellor will be able to discuss the risks with you in more depth. The diagram explains how the faulty gene is passed on from men with X-linked conditions.
What are the risks to my children if I am a female carrier of an X-linked condition?
Female carriers of X-linked conditions have one X chromosome containing a faulty copy of the gene, and one X chromosome containing a healthy copy. In most cases, they have healthy vision.
If you are a female who already has a child with a confirmed diagnosis of an X-linked condition, you are almost certainly a carrier. The only exception to this would be if the fault has occurred for the first time in your child (a de novo mutation – see Why genes matter).
If you are a female who has a family history of X-linked sight loss, you might already have had a genetic test and found out that you are a carrier.
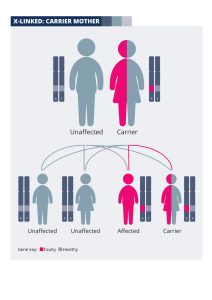 With each pregnancy, a mother who is a carrier has a 50% (one in two) chance of passing the X chromosome with the faulty copy of the gene onto her baby, and a 50% chance of passing on the X chromosome with the normal copy. If the baby is a boy, his other sex chromosome will be a Y from his father, which will not contain another copy of the gene. Therefore a boy inheriting the X chromosome with the faulty gene from his mother will be affected by sight loss.
With each pregnancy, a mother who is a carrier has a 50% (one in two) chance of passing the X chromosome with the faulty copy of the gene onto her baby, and a 50% chance of passing on the X chromosome with the normal copy. If the baby is a boy, his other sex chromosome will be a Y from his father, which will not contain another copy of the gene. Therefore a boy inheriting the X chromosome with the faulty gene from his mother will be affected by sight loss.
If the baby is a girl, she will have inherited an X chromosome from her father, which will contain a healthy copy of the gene. If she inherits the X chromosome with the faulty gene from her mother, she will be a carrier; she is likely to be unaffected or more mildly affected.
Therefore, for each male pregnancy there is a 50% chance of the baby boy being affected by sight loss, and for each female pregnancy there is a 50% chance of the baby girl being a carrier.
A genetic counsellor can discuss these risks with you in more depth. If you are planning a pregnancy, they can also talk to you about options for testing during pregnancy and access to reproductive technology such as pre-implantation genetic diagnosis (see Genetic counselling). These options will only be available if you or the child living with sight loss has already had a genetic test.
The diagram explains how X-linked conditions are passed on from a couple where the mother is an unaffected carrier:
I am a female with an affected brother and am planning a family; will my children be at risk from sight loss?
If your brother (or half-brother if you have the same mother) has X-linked retinal disease, there is a 50% chance that you will carry the faulty gene and your sons will be at risk of sight loss. If your brother has had a genetic test result, this will provide you with options to find out whether you are indeed a carrier. You can ask your GP for a referral to your local regional genetics service for genetic counselling and testing.
If you are a carrier, the risks to your children will be as described in the previous section: What are the risks to my children if I am a female carrier of an X-linked condition? Your genetic counsellor will be able to discuss these risks in more depth and talk to you about options for family planning.
Research into treatments for X-linked retinal disease
Like autosomal recessive conditions, X-linked conditions are often the result of genes producing a protein that simply doesn’t work properly, so in some cases it may be possible to overcome the problem by injecting healthy copies of the gene into the back of the eye; this is known as gene replacement therapy. Retinal cells can use these healthy genes to produce normal protein, restoring function and enabling vision to be maintained or even improved.
This approach is being investigated for XLRP caused by mutations in the RPGR gene and is currently at the clinical trial stage. The same approach to treating XLRP caused by mutations in the RP2 gene is also being explored, but is still being tested in laboratory models.
