New stem cell treatment gains approval to enter US clinical trials
New stem cell treatment gains approval to enter US clinical trials
Search results
New stem cell treatment gains approval to enter US clinical trials

It’s time to reflect, celebrate and look to the future... and you’re invited. Join us for an unforgettable evening as we celebrate the culmination of our 50th anniversary year.

The cover of this edition celebrates the success of our amazing London Marathon team. Together they have raised more than £43,000.
Next month, Anisha and Sheena will take on The Thames Path Challenge. Together, they explain why they decided to join #TeamRetinaUK.
Early 2020 marked an important milestone for the Retina UK community, when the first person with an inherited retinal condition received NHS treatment to potentially slow or even stop the progression of their sight loss.
Disulfiram (Antabuse), FDA-approved for deterring alcohol, is in a phase 1 trial at the University of Washington to improve vision in retinitis pigmentosa.
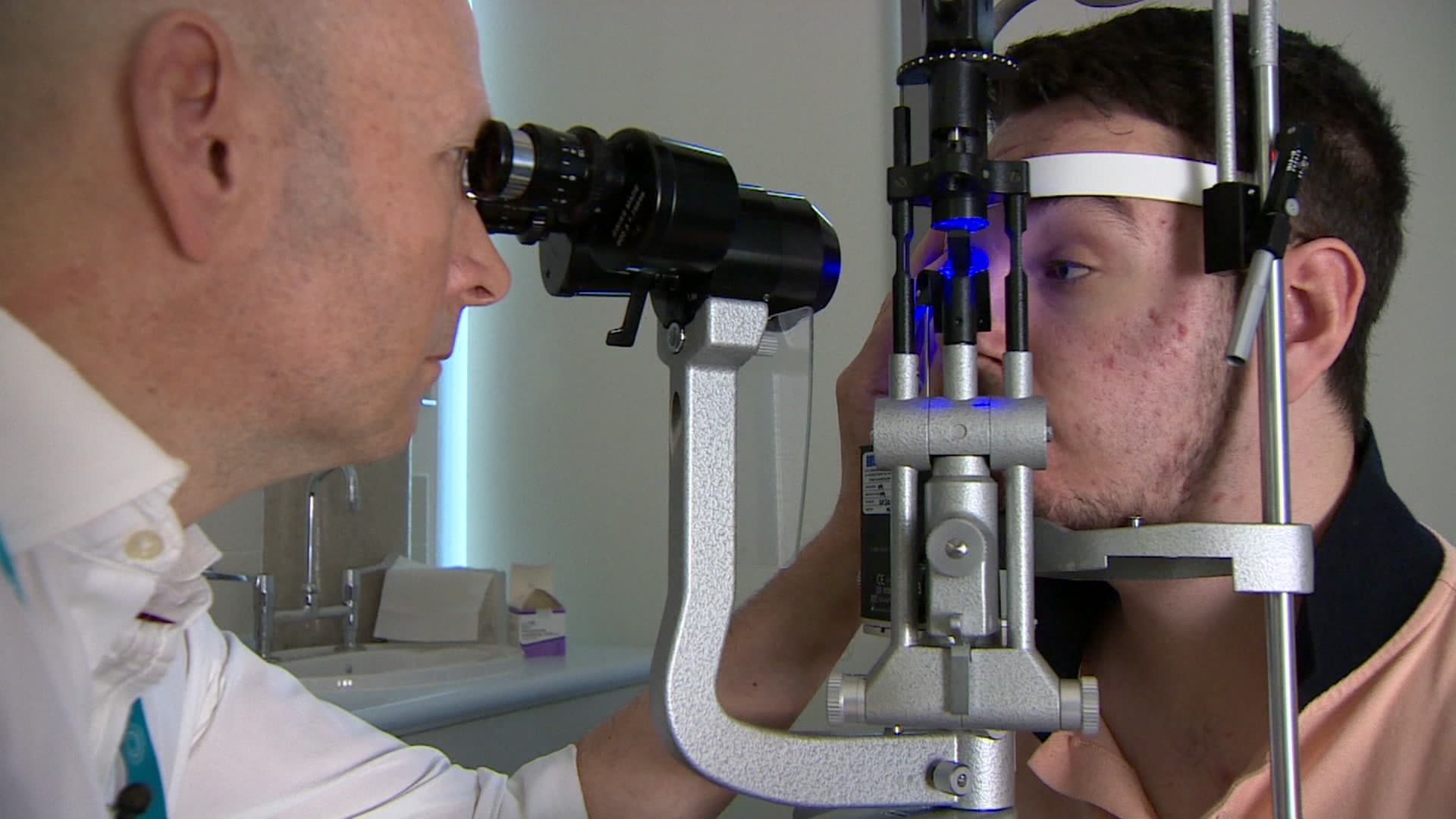
In January 23-year-old Jake Ternent became the first person with an inherited sight loss condition to be treated in the UK with Luxturna (voretigene neparvovec) for Leber congenital amaurosis (LCA).

There are many ongoing clinical and laboratory studies around the world, exploring innovative approaches to treating inherited sight loss.
It was previously believed that female carriers of X-linked inherited retinal diseases (IRDs) like X-linked retinitis pigmentosa (RP and Choroideremia) remained unaffected by sight loss.
Introducing Splice Bio, a genetic medicines company with some exciting developments for Stargardt’s patients.